elisa test generation|elisa hiv test : inc The enzyme-linked immunosorbent assay (ELISA) (/ɪˈlaɪzə/, /ˌiːˈlaɪzə/) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand (commonly a protein) in a liquid sample using antibodies directed against the ligand to be measured. EL. Poteau contrecollé Pin rouge du Nord traité classe 4 140x140mm longueur 3,00 mètres Réf 514640 de la marque PROTAC sur Pointp.fr. Retrait en agence sous 2h. 850 Agences et 150 .
{plog:ftitle_list}
Avante Animal Health now offers the Prestige 210006. The Prestige 210006 is one of the worlds largest selling and cost effective automatic autoclaves available. Sold in 80 countries .
The enzyme-linked immunosorbent assay (ELISA) (/ɪˈlaɪzə/, /ˌiːˈlaɪzə/) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand (commonly a protein) in a liquid sample using antibodies directed against the ligand to be measured. EL."Like any screening test, a reactive result (a preliminary positive result) must be verified with confirmatory tests." These tests may also be referred to as “fourth-generation” tests or as an ELISA (enzyme linked immunosorbent assay). The first- and second-generation laboratory tests are no longer in use.
Bio-Rad's fourth-generation GS ELISA was FDA cleared in 2011. The GS ELISA was evaluated on 9,150 specimens and showed 100% sensitivity and 99.9 to 100% specificity . Siemen's ADVIA fourth-generation assay, to be used on the Centaur instrument, was approved in 2015. . Like the fifth-generation assay, this test would benefit from a different .The enzyme linked immunosorbent assay (ELISA) is a powerful method for detecting and quantifying a specific protein in a complex mixture. Originally described by Engvall and Perlmann (1971), the method enables analysis of protein samples . Enzymatic signal generation requires the catalysis of a substrate to produce a colored or fluorescent .Although ELISA is a powerful and well-characterized application, attempting to develop and optimize a specific assay can be difficult. The method involves the assembly of a large immune complex with multiple components. These intricacies can lead to a failure to produce an ELISA signal; optimization of an assay is essential.
ELISA, short for Enzyme-Linked Immunosorbent Assay, is a widely used laboratory technique that detects and measures the presence of specific antibodies or antigens in a sample. It involves the binding of target molecules (antibodies or antigens) to a solid surface, followed by the addition of enzymes or fluorescent markers to generate a detectable signal. ELISA is . Enzyme Linked Immunosorbent Assay (ELISA) is a very sensitive immunochemical technique which is used to access the presence of specific protein (antigen or antibody) in the given sample and it’s quantification.
Third generation tests are an ELISA that looks for an antibody alone. Fourth generation tests use both of the above methods to look for both antibodies and antigens. . What does the number after the HIV ELISA test result mean? Some people are given test results which say something like ‘non-reactive (OD: 0.219)’. This number at the end is .
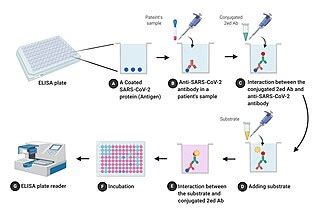
Over the past three decades, ELISA or enzyme-linked immunosorbent assay, has become vital to many areas of research and has shown to have many applications—from detecting food and environmental contaminants to screening for HIV and SARS-CoV-2 (COVID-19) antibodies ELISA overview. ELISA is a method used to quantitatively detect an antigen (i.e., toxin or foreign .Enzyme-Linked Immunosorbent Assay (ELISA) is a widely used laboratory technique that employs antibodies and enzymes to detect and quantify various biomolecules in biological samples. This method involves the use of a substrate that changes color when acted upon by the enzyme, resulting in a visible signal that indicates the presence and quantity of the target [.] ELISA test results, what does a positive ELISA test tell you? ELISA results may be interpreted quantitatively, qualitatively or semi-quantitatively. In a quantitative assay, a serial dilution of a known standard is used to enable the generation of a standard curve, normally of optical density (OD) versus concentration. From this, the precise .
Updated Nomenclature for HIV Serologic Tests. In the United States, the Food and Drug Administration (FDA)-approved HIV serologic tests have historically been categorized as first-, second-, third-, and fourth-generation tests, based on evolving techniques and significant improvement in assay sensitivity.
ELISA. The enzyme system of ELISA consists enzyme which is labeled to a specific antibody or antigen and a chromogenic substrate that is added after the antigen-antibody reaction. The substrate is hydrolyzed by the enzyme attached to antigen-antibody complexes. An ELISA test uses components of the immune system (such as IgG or IgM antibodies) and .The fourth-generation assay is less expensive, easy to perform, and is an automated assay. . most particular and common immunoassay utilized for HIV antigen-antibody detection is the enzyme-linked immunosorbent assay (ELISA). The technique has developed from tests of viral-lysate-based IgG to first-generation tests (window period, 40–50 . To test for HIV, a series of blood screenings may be done, including one called the ELISA test. In case of a positive result, the ELISA test is typically followed by an HIV differentiation assay . Background/Objectives Guidelines for optimized HCV screening are urgently required in Africa, especially for patients infected with HIV, who sometimes show false positive or false negative reactivity in anti-HCV antibody assays. Here, we assessed the usefulness of a fourth-generation HCV Ag-Ab ELISA for the identification of active HCV infection in HIV .
We would like to show you a description here but the site won’t allow us.
Evolution of HIV ELISAs: The ‘first generation ELISA’ for HIV infection used whole viral lysates from cultures of HIV-1 from lymphocytes [28].They suffered from the main limitation of a high rate of false positivity in low risk populations, due to reactions of serum antibodies to cellular contaminants such as those from major histocompatibility complex [29].Fourth generation Enzyme linked lmmunosorbent Assay (ELISA) for the detection of antibodies to HIV1/2 & O subtypes and p24 antigen in human serum or plasma. CAT Nos. Pack Size; 404030096: 96 Tests . Fourth generation Chemiluminescence assay for the detection of antigens & antibodies to HIV 1&2 virus in human serum or plasma. CAT Nos. Pack .Enzyme-linked immunosorbent assay (ELISA) is an immunological technique extensively used in research and clinical laboratory settings to quantitatively identify a specific protein (i.e., the antigen or biomarker) in a biological matrix while relying on the principle of the specific binding interaction between the antigen and the antibody against the antigen of interest .
A previous study has shown that the Genscreen ULTRA HIV Ag‐Ab ELISA test (EVOLIS BioRad) used as reference in this study for the detection of HIV could present false positives.21 Also, the Elecsys HIV combi PT kit used by COBAS 6000 e601 was developed to have a higher sensitivity allowing an early detection of HIV in the seroconversion phase .
Aim:To compare sensitivity and specificity of rapid diagnostic test (RDT) with fourth generation ELISA Material and Method: This study was conducted in the Department of Microbiology at Atal .
The Enzyme Linked Immunosorbent Assay (ELISA) is one of the most sensitive immunoassays available. The typical detection range for an ELISA is 0.1 to 1 fmole or 0.01 ng to 0.1 ng. It is a plate-based assay technique designed for .The most widely used ELISA assay format is the sandwich ELISA assay, which indirectly immobilizes and indirectly detects the presence of the target antigen. This type of capture assay is called a “sandwich” assay because the analyte to be measured is bound between two primary antibodies, each detecting a different epitope of the antigen . Figure 1. Time course of HIV-1 RNA, HIV antibodies, and HIV p24 antigen detection for 1st-, 2nd-, 3rd-, and 4th-generation HIV assays. 3 The principal difference between the BioPlex 2000 HIV Ag-Ab assay and currently available fourth-generation HIV assays is the ability to discriminate between HIV-1 and HIV-2 infection in a single assay.
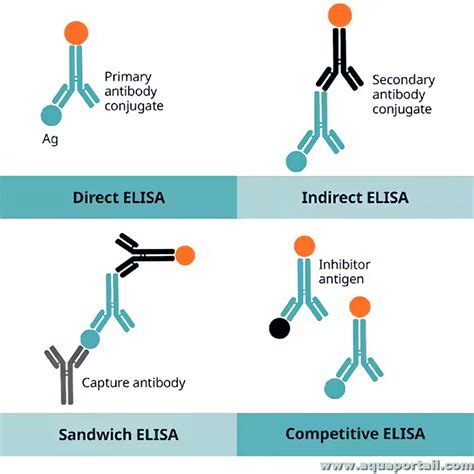
ELISA (Enzyme-Linked ImmunoSorbent Assay) is an immunologic technique used to detect the presence and concentration of an antigen or antibody in a sample. The power of an ELISA is based on the extreme specificity of the antigen-antibody interaction. ELISAs have wide-ranging applications, especially as medical diagnostic tools. Figure 1. ELISA .ELISA is a plate-based assay that uses antibodies to specifically detect and quantify the amount of a target antigen in a liquid sample. +1 (314) 370-6046 or . Integration: Combining ELISA with other technologies, such as mass-spectrometry or next-generation sequencing; ELISAs are now commonplace and invaluable in medical diagnostics, drug .4th Generation ELISA Test. Detection of HCV Core Antigen and Antibodies to HCV(Core ,NS3,NS4 & NS5) in Human serum/Plasma; Significant reduction in window period by 5-7 weeks; Detects all 8 HCV Genotypes; Colour coded reagents to monitor the procedural steps; Assay procedure: 150 minutes;
elisa test wikipedia
abcam p24 elisa kit
abcam pge2 elisa kit
$3,909.95
elisa test generation|elisa hiv test